 Even
if there is no patent on a medicine, generic versions still cannot be approved
by the Pharmacy and Poisons Board in Kenya for a period of five years. The cost of the branded drug covered by the exclusivity clause can then be raised multiple times.
Even
if there is no patent on a medicine, generic versions still cannot be approved
by the Pharmacy and Poisons Board in Kenya for a period of five years. The cost of the branded drug covered by the exclusivity clause can then be raised multiple times.
On
fourteenth January of this year, President William Ruto and His Highness Sheikh Mohamed bin Zayed Al Nahyan, President of the United
Arab Emirates (UAE) signed a Comprehensive Economic Partnership Agreement
(CEPA) between their respective countries.
The agreement has been hailed as being the first agreement of its kind signed by the UAE with a mainland African country. According to the Ministry of Foreign Affairs, it represents a “transformative step in enhancing trade, investment, and economic cooperation”.
However, for trade agreements, experts will tell you that the devil is always in the details. One could go on for a while about the Kenya/UAE CEPA but to begin with, it must be acknowledged that is a strange agreement even for one related to trade.
Standard trade agreements usually have market access provisions where tariffs on goods are gradually reduced. This one does not. One is tempted to ask what benefit the country is going to get for all the concessions it is giving under the CEPA. If it doesn’t dismantle tariffs, then it means Kenya can still trade under the World Trade Organizations (WTO) framework and still export to the UAE without having to open up under the CEPA with minimal benefits.
Putting aside all the implications for the agreement, one must home in on the potential impact the trade agreement has on the health sector. It may not be possible to examine the entire health sector but let’s take the disease burden for HIV/Aids, tuberculosis and cancer by way of example.
Kenya faces a significant disease burden that costs the country a lot of money. According to the National Aids and STIs Control Programme, over 1.4 million people live with HIV/Aids in Kenya.
The country has about 18,000 annual HIV/Aids related deaths and has about 1.2 million people on antiretroviral therapy accounting for about 86 per cent of people living with HIV. This is without factoring in the cancelation of the United States Presidential Emergency Plan for Aids Relief (PEPFAR) under the recently dismantled United States Agency for International Development.
In the case of Tuberculosis (TB), estimates put the annual cases of TB infections at about 133,000 cases for all forms of TB cases every year. In 2022, the country reported 90,841 TB cases accounting for about 68 per cent case detection. Drug-resistant TB cases are about 1,200 annually. The TB-HIV co-infection rate stands at 20 per cent with that percentage of TB patients also being HIV-positive. The TB deaths in 2022 were about 12,000. For cancer, the case is also dire. According to the Kenya National Cancer Registry, the country has an estimated 42,000 new cancer cases every year. Annual deaths are 27,000.
The treatment for these diseases is not cheap. In the case of cancer, for example, the cost of generic chemotherapy drugs such as Doxorubicin, Cyclophosphamide or Paclitaxel is estimated at between Sh5,000- 30,000 per dose depending on the drug and dosage. In the case of branded or targeted therapy drugs such as Herceptin, Trastuzumab, Keytruda or Pembrolizumab, the cost is estimated at between Sh100,000-500,000 per dose while some can exceed Sh1 million for immunotherapy drugs. For hormonal therapy drugs such as Tamoxifen or Letrozole, the cost is between Sh500 – 10,000 per month.
Cancer patients also need pain management and supportive drugs such as morphine and anti-nausea medications, whose cost is estimated at between Sh200 -10,000 per month depending on brand and dosage. These are heavy costs for patients at a time when the economy is not doing very well.
The Kenyan Government has been subsidising some of these drugs to make them affordable to patients. When considered against a backdrop of a far-reaching fiscal consolidation programme by the International Monetary Fund (IMF), these subsidies may not be kept for a long time.
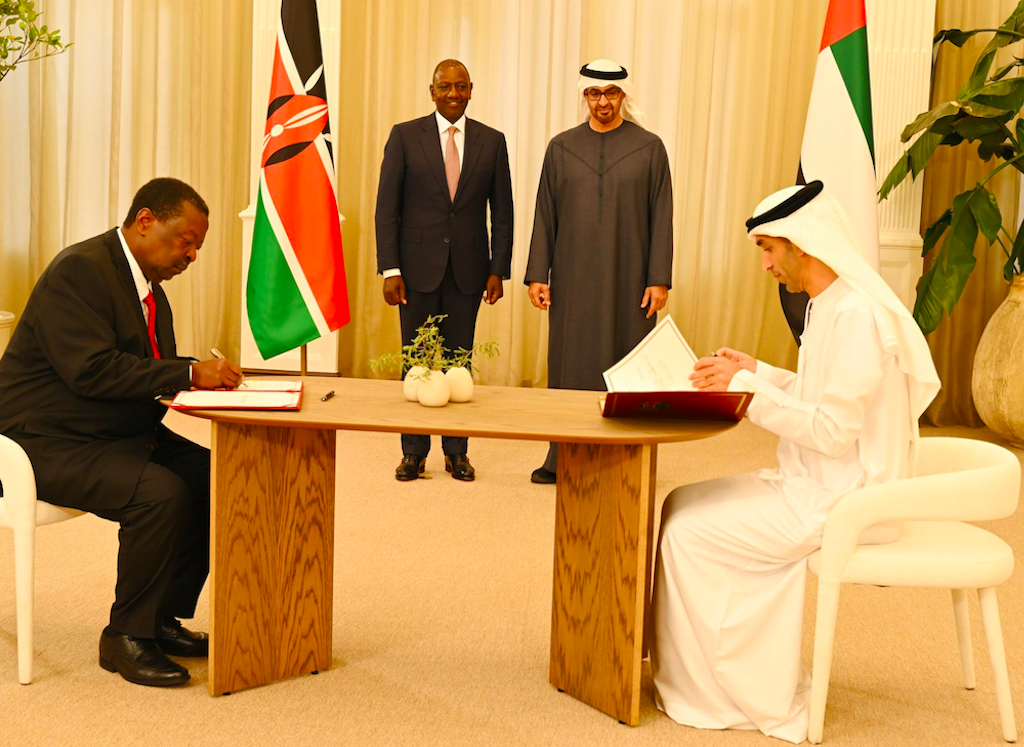 President William Ruto and His Highness Sheikh Mohamed bin Zayed Al Nahyan, President of the United Arab Emirates (UAE), when the two governments signed a Comprehensive Economic Partnership Agreement (CEPA) on January 14, 2025.
President William Ruto and His Highness Sheikh Mohamed bin Zayed Al Nahyan, President of the United Arab Emirates (UAE), when the two governments signed a Comprehensive Economic Partnership Agreement (CEPA) on January 14, 2025.
When the cancellation of the PEPFAR programme by the US Government
is added to the mix, then you have a powerful cocktail of trouble coming for
the health sector in terms of managing the healthcare costs for the country
even before you put the Kenya/UAE CEPA into play with its significant impact on
healthcare costs. Now picture this: the Government has actually signed a trade
agreement that is going to make these drugs even more costly!
I am shocked to see that this Kenya-UAE CEPA has included clauses that entail obligations for market exclusivity and linkage. In simple terms, market exclusivity places an obligation for market exclusivity for a period of five years for a particular medicine from the latest possible date for both the information in the dossier and the fact of the marketing approval.
This means that even if there is no patent on a medicine, for example, because it is not a new invention like insulin, and therefore not eligible for patent protection, generic versions still cannot be approved by the Pharmacy and Poisons Board as safe and effective and so reach Kenyan patients for a period of five years. A hard linkage obligation on the other hand, prevents compulsory licences from being effective.
A compulsory license is when a government allows someone else to produce a patented product (such as a medicine) or process without the consent of the patent owner or plans to use the patent-protected invention itself. It is one of the flexibilities in the field of patent protection included in the WTO’s Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS). It should be noted that the India-UAE CEPA, which was signed in 2022, does not have provisions on exclusivity and linkage because India expressly rejected their inclusion to protect its public health interests.
There are countless examples from across the globe on how these provisions have made medicines expensive. Colombia has had data exclusivity legal obligation between 2002. In a 2012 study, it was found that these data exclusivity requirements would cost Colombia an additional US$ 396million in additional expenses for its public health system from 2003-2022.
Another real life example of the impact of data or market exclusivity is when an old medicine to treat gout (Colchicine) was given three years of market exclusivity in the USA as a new indication for this medicine and the company which received it sued to remove existing versions of colchicine from the market and then raised the price by more than 50 times adding $50 million per year to the cost of providing this medicine in the USA. It was also given seven years of market exclusivity for colchicine to treat a rare disease (familial Mediterranean fever), even though this was already a known use of the medicine. This is the reality that Kenya faces if the Kenya/UAE CEPA is ratified in its current form.
Fortunately for Kenya, the 2010 Constitution has put in place fail-safe mechanism to protect against such decisions. Article 21 of the Kenyan Constitution places a fundamental duty on the State and all its organs to ensure that the rights and fundamental freedoms enshrined in the Bill of Rights are upheld.
This duty encompasses observing, respecting, protecting, promoting, and fulfilling the rights and freedoms. Specifically, Article 21 includes the right to health within the scope of rights and freedoms that the State must uphold. The right to health, as recognized in the Kenyan Constitution, includes the right to the highest attainable standard of health, which encompasses access to healthcare services, including reproductive health care.
Since all treaties have to be ratified by Parliament before coming into force in Kenya, the ball is now in the court of the legislature to observe and respect the Constitution and protect Kenyans from this predatory agreement. The Parliamentary Caucus on Business and the Economy has been a force for good during such moments.
Following the controversy during the ratification of the Kenya/United Kingdom EPA, the Caucus proposed amendments to the Parliamentary Standing Orders with respect to ratification of treaties especially given the weakness in the Treaty-Making and Ratification Act with respect to economic treaties. With these new standing orders, Parliament can approve the ratification of the agreement, approves ratification with reservations, or rejects the ratification. Therefore, I propose that our legislature proposes reservations to the Treaty and insist on removal of obligations that go beyond the TRIPS Agreement and insist on the inclusion of a market access chapter. Over to Parliament!
Edgar Odari is the Executive Director, Econews Africa







![[PHOTOS] Guardian Angel bus catches fire in Kikuyu](/_next/image?url=https%3A%2F%2Fcdn.radioafrica.digital%2Fimage%2F2025%2F04%2F58287f0a-f201-4a78-87f0-6f147ad8ba8a.jpg&w=3840&q=100)



