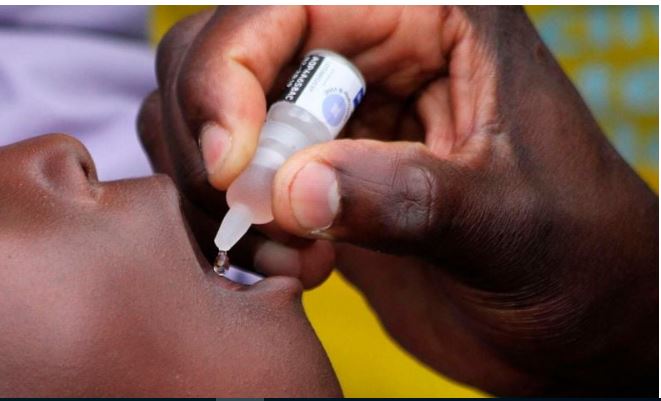
Kiambu Director of Health Dr Hillary Kagwa has firmly refuted reports and propaganda circulating about the polio immunisation programme, assuring Kenyans that the vaccines are safe and approved by the World Health Organization (WHO).
During a stakeholder sensitisation forum held over the weekend to discuss the rollout of the second round of the Polio immunisation campaign in nine counties in Kenya, Dr. Kagwa emphasized that the vaccine was considered a noble one medically, while there have been rare instances of children experiencing side effects related to their genetic makeup, these effects were generally manageable and in a majority of case often resolved on their own.
“There is considerable misinformation surrounding the polio vaccine. These vaccines have been approved by the World Health Organization (WHO) and confirmed to be safe. It's important to note that adverse effects following immunization can occur with any vaccine or medication, as individuals have varying genetic makeup, which can influence their responses,” he explained.

“We aim at vaccinating approximately 300,000 children under the age of 5. Those who received their vaccinations during the initial phase in October can participate in this second round to enhance their immunity,” said Dr Kagwa.
Stakeholders from AMREF who were also present at the event underscored the significance of the vaccination initiative, urging parents and guardians to ensure their children receive the necessary immunizations.
This follows the Ministry of Health's announcement on Friday that the October 2024 vaccination campaign had led to the deaths of two children.
Director General for Health Dr Patrick Amoth announced that the Ministry had received 23 reports of post-vaccination events related to the campaign, indicating that the Ministry had consulted with the Kenya National Vaccines Safety Advisory Committee (KNVSAC), which convened on October 23 and 24 to evaluate all the reported cases.
He explained that among the 23 reported and investigated cases, 16 were classified as non-serious, presenting symptoms such as generalized itchy rash, fever, conjunctivitis, body rashes, abdominal pain, and diarrhoea. In contrast, 7 cases were deemed serious, featuring symptoms such as fever, convulsions, and weakness in the lower limbs.

Dr. Amoth noted that most cases of Adverse Events Following Immunization (AEFI) were categorized as coincidental, emphasizing the significance of thorough causality assessments to accurately differentiate between coincidental events and genuine vaccine-related reactions stating that the distinction was crucial for maintaining public trust in the safety of the immunization program.
He observed that over 3.6 million healthy children received vaccinations during the campaign, indicating that the vaccines were largely safe.